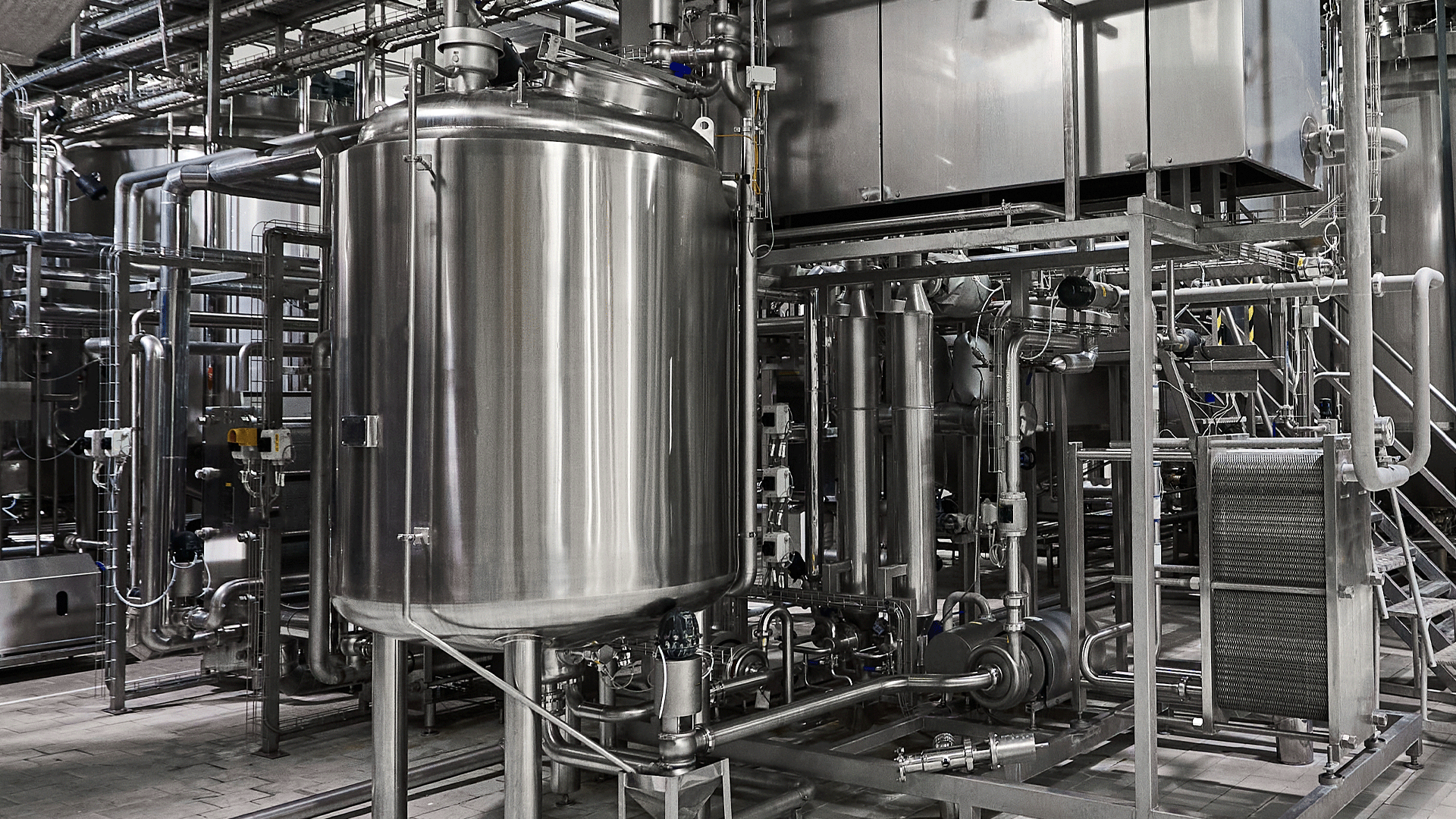
At Sanitary Fittings, we understand that maintaining optimal hygiene and efficiency in manufacturing processes is paramount. One integral aspect of this is the Clean-in-Place (CIP) system. In this blog post, we will delve into the fundamentals of CIP, exploring its significance, components, and applications. CIP is also the opposite of COP, or clean-out-of-place.
What is Clean-in-Place (CIP)?
Clean-in-Place, commonly known as CIP, is a method employed in many industries to clean the interior surfaces of pipes, vessels, process equipment, and associated fittings without disassembling them. The primary goal of CIP is to eliminate contaminants, residues, and microorganisms, ensuring the equipment is sanitary and ready for the next production cycle.
The Components of CIP:
- Cleaning Agents:
CIP systems use a variety of cleaning agents, including detergents, acids, and sanitizers, depending on the nature of the residues to be removed. These cleaning agents are selected to optimize the removal of specific contaminants while ensuring compatibility with the equipment materials. - Pumps:
CIP systems utilize pumps to circulate cleaning solutions through the processing equipment. High-pressure pumps are often employed to ensure effective cleaning by reaching all surfaces and crevices. - Heat Exchangers:
In some cases, heat exchangers are integrated into CIP systems to enhance the cleaning process. Thermal energy can improve the efficiency of cleaning agents, especially in removing stubborn residues. - Control Systems:
CIP systems are equipped with advanced control systems that manage the entire cleaning process. Automation ensures consistency, efficiency, and precise control over parameters such as temperature, flow rate, and cleaning agent concentration.
The CIP Process:
- Pre-Rinse:
The CIP process typically begins with a pre-rinse to remove loose contaminants. This step helps in preparing the equipment for the main cleaning phase. - Main Cleaning Cycle:
During the main cleaning cycle, the selected cleaning solution is circulated through the system at high velocity and temperature, effectively breaking down and removing residues. - Post-Rinse:
A post-rinse follows the main cleaning cycle to ensure the removal of any remaining cleaning agents and contaminants. This step is crucial for achieving the desired level of cleanliness. - Validation:
CIP systems often include validation steps to confirm the effectiveness of the cleaning process. This may involve testing for residual contaminants or conducting visual inspections.
Applications of CIP:
- Food and Beverage Industry:
CIP is widely used in the food and beverage industry to clean processing equipment such as tanks, pipes, and heat exchangers, ensuring compliance with hygiene standards and is very common in the brewing industry. - Pharmaceutical Industry:
In the pharmaceutical sector, CIP plays a critical role in maintaining the cleanliness of manufacturing equipment, preventing cross-contamination, and meeting stringent regulatory requirements. - Biotechnology and Dairy Industry:
CIP is also employed in biotechnology and dairy production to sanitize equipment and maintain the integrity of the final products.
Conclusion:
Clean-in-Place is a pivotal process in modern industrial operations, ensuring that equipment is not only efficient but also meets the highest standards of hygiene. As industries continue to evolve, the role of CIP in maintaining cleanliness, efficiency, and compliance with regulatory standards will become more important. Understanding the principles and applications of CIP is essential for professionals in various fields in order to maintain efficient production processes. We are happy to offer our full line of washdown stations, hoses, nozzles, and washdown racks.